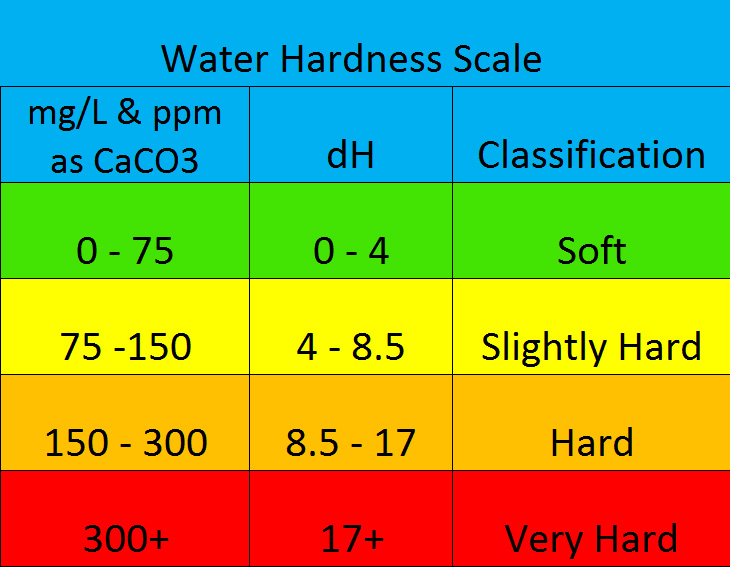
 Water hardness is a critical factor in keeping fish and an understanding of this will make it easier to maintain a healthy environment in the aquarium . Water hardness refers to the amount of dissolved minerals in a body of water. In nature water filters through rocks, collecting minerals and other sediments along its journey downstream which determines the hardness of the water for that particular area. Soft water contains less mineral content while harder water contains more. Hardness is measured by Carbonate Hardness(KH) and General Hardness(GH). It is most commonly referred to in German degrees(dKH, dGH, dH), milligrams per litre(mg/L) or parts per million(ppm) of Calcium Carbonate(CaCO3).
Water hardness is a critical factor in keeping fish and an understanding of this will make it easier to maintain a healthy environment in the aquarium . Water hardness refers to the amount of dissolved minerals in a body of water. In nature water filters through rocks, collecting minerals and other sediments along its journey downstream which determines the hardness of the water for that particular area. Soft water contains less mineral content while harder water contains more. Hardness is measured by Carbonate Hardness(KH) and General Hardness(GH). It is most commonly referred to in German degrees(dKH, dGH, dH), milligrams per litre(mg/L) or parts per million(ppm) of Calcium Carbonate(CaCO3).
Carbonate Hardness(KH) or Alkalinity refers to the amount of carbonate and bicarbonate ions in the water. Carbonates and bicarbonates are salts of carbonic acid. Carbonic acid is formed when carbon dioxide gas is dissolved in water. The higher the KH, the more stable the pH of the water will be. This is what is referred to as the Buffering capacity of the water, which is the waters ability to maintain and hold a stable pH. Carbonate Hardness can also be referred to as temporary hardness. This is due to the fact that it can be removed through boiling. Carbonate hardness has a direct relationship with the pH of water and when the hardness is high, your pH will also be high and when the hardness is low, the pH will follow and be low. 1 dKH equals 17.9ppm or 17.9mg/L
General Hardness(GH) is the measure of dissolved minerals, mainly calcium and magnesium in the water. This is what is commonly referred to as soft or hard water. These minerals hold an electric charge that helps with proper osmoregulation of fish. A higher GH of around 6 – 10dGH is best for a majority of fish and plant life as the ions provide essential electrolytes that do not come from a simple water change. It is better to have a higher GH than a lower GH as this helps to supplement these ions into the aquarium as in nature this happens naturally from the constant flow of fresh water. Using a GH Booster or mineral supplements will help maintain proper amounts of mineral ions that over time are depleted in the aquarium. General Hardness is also referred to as Total hardness or Permanent hardness as it cannot be broken down by boiling. 1 dGH equals 17.9ppm or 17.9mg/L
Increasing Hardness
Using chemical buffers is an easy way of raising the hardness of the water to a desired level. Many major brands have buffers that will help to raise KH & GH.
Sodium Bicarbonate – Baking Soda will raise the KH of the water.
Crushed Coral is made up mostly of Calcium Carbonate which is ideal for raising water hardness. Texas holey rock is limestone rock which will provide calcium and other essential minerals to leech into the water of your aquarium. These are released into the water at a more gradual rate than that of chemical addition.
Decreasing Hardness
To soften hardwater the calcium and magnesium ions need to be removed.
Reverse Osmosis(RO) or a De ionized (DI) Filter will reduce the hardness of the water. You may want to mix RO or DI water with some normal tap water to get the desired hardness. These filters will also strip out all minerals from the water which are vital for the health of your fish leaving it mineral free. Re-mineralizing the water is essential so the fish can get what they need for proper osmotic function to survive.
Rainwater is also used as a way of lowering the hardness of the water. Again you will want to re-mineralize this water and add buffers to stabilize.
Driftwood will soften your aquarium water as it releases tannins gradually over time. Some driftwoods like Mopani are heavy leeching, so this will have a increased effect on your water parameters.
Peat is another very effective way of softening your aquarium water. It will bind to the calcium and magnesium ions and slowly release tannic acids. Peat can vary in strength and quality and can be unpredictable due to the fact that change can be minimal or extreme. It is better to mix this into a bucket first and then add the required amount to monitor exactly what levels are needed.
An aquarium with Carbonate Hardness below 3dkH should be monitored more closely as the pH in the aquarium is susceptible to drop unexpectedly due to a low buffing capacity. Over time hardness will be depleted due to the many processes in the aquarium that will produce acids e.g. High feeding rates, a high fish population or long periods between water changes. Increasing the hardness can easily be done by adding more of any form of calcium carbonate, water changes with a higher alkalinity and/or by adding more buffering chemicals to replenish the desired levels that are needed for the aquarium.
